40 fda requirements food labels
Food Labeling 101 - FDA Regulations Guide [2022] Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings › cosmetics › potential-contaminants1,4-Dioxane in Cosmetics: A Manufacturing Byproduct | FDA Mar 03, 2022 · FDA infrormation on 1,4-dioxane, a contaminant that may occur in trace amounts in certain cosmetics.amounts in certain cosmetics. The following information has been compiled from responses to ...
› food › food-labeling-nutritionMenu Labeling Requirements | FDA - U.S. Food and Drug ... The menu labeling requirements apply to restaurants and similar retail food establishments that are part of a chain with 20 or more locations. In addition, they must be doing business under...

Fda requirements food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.9 Nutrition labeling of food. (a) Nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is... FDA Food Labeling Exemptions - LabelCalc A lot of people get tripped up on the rounding rules for the nutrition facts panel, others get confused about nutrient content claims, and some have trouble understanding label size requirements. The one thing, however, that almost all food manufacturers struggle with is understanding the FDA food labeling exemptions. › food › food-labeling-nutritionFood Allergies | FDA - U.S. Food and Drug Administration Jun 23, 2022 · The FDA’s “Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food” rule (CGMP & PC rule, 21 CFR part 117) establishes requirements applicable ...
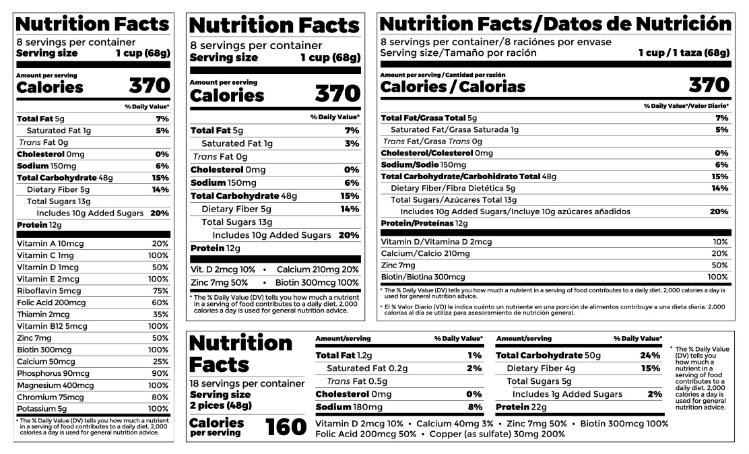
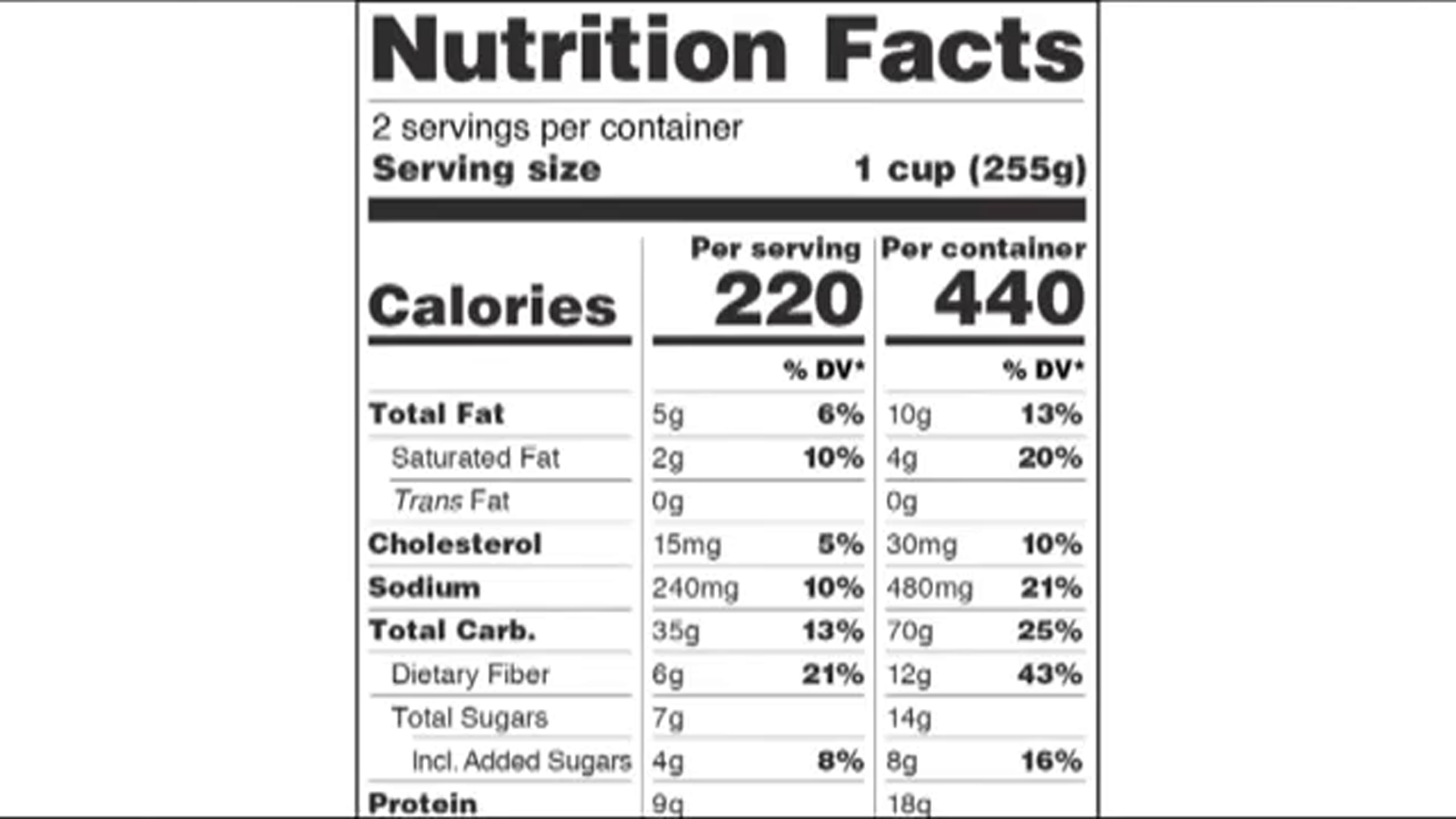
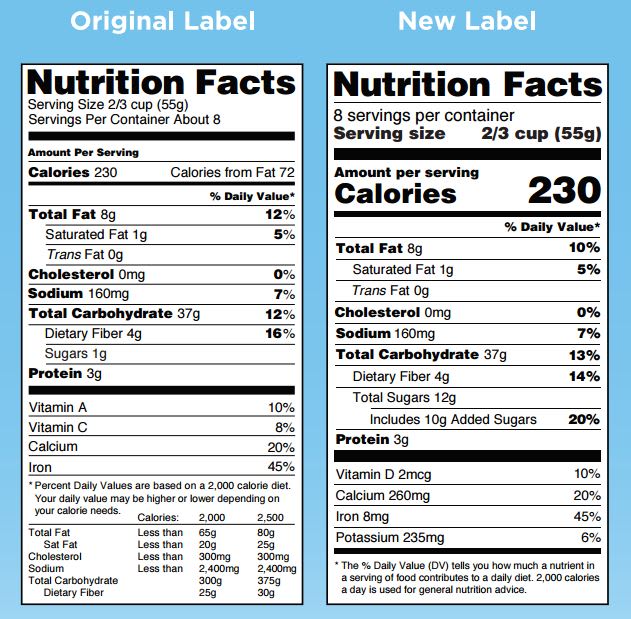
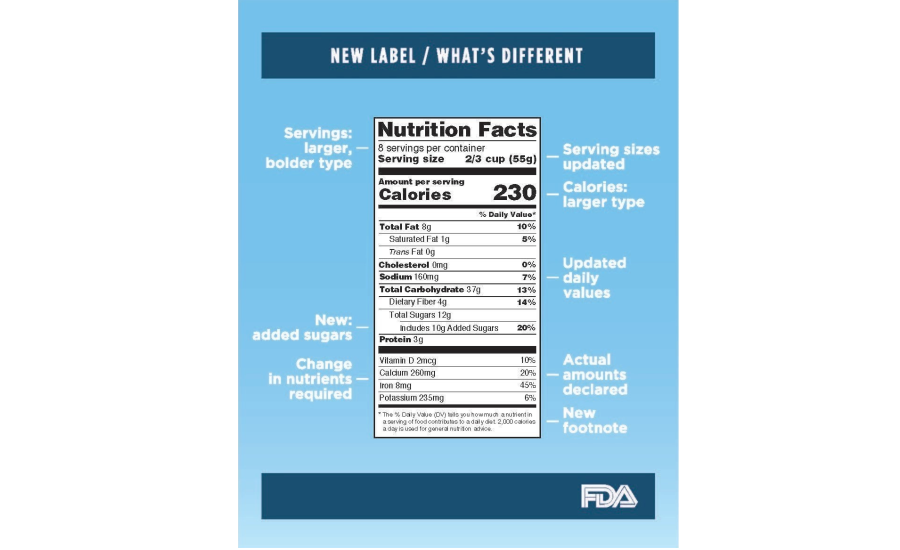
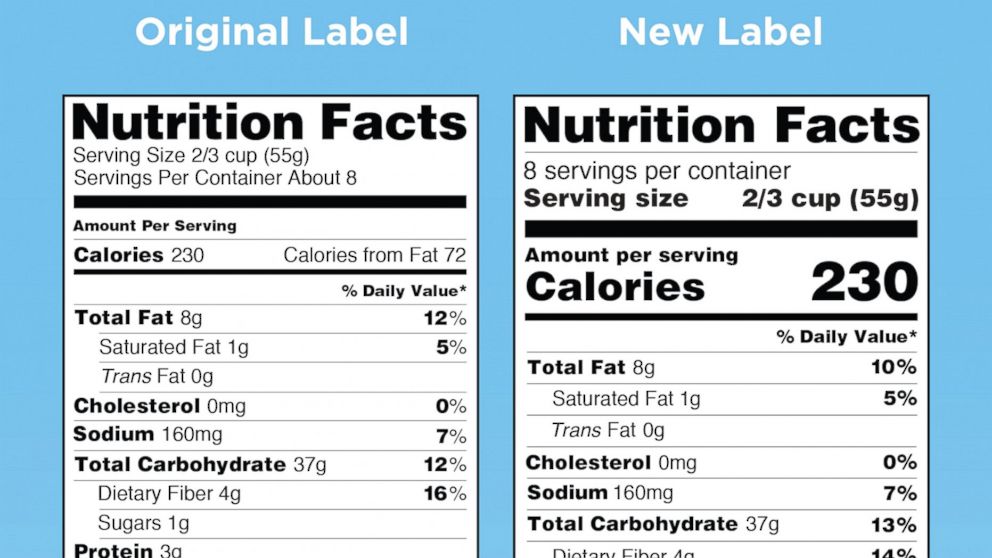
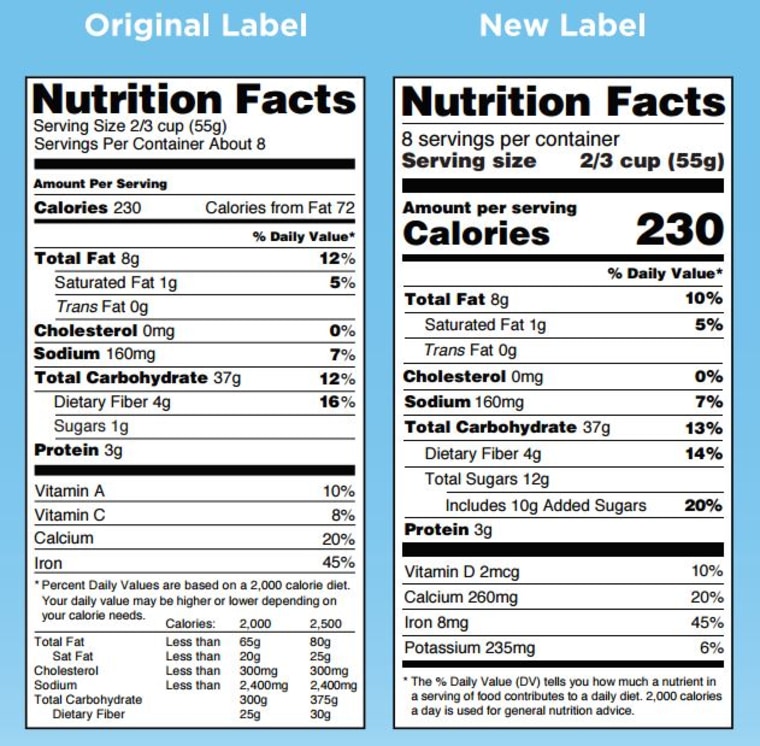
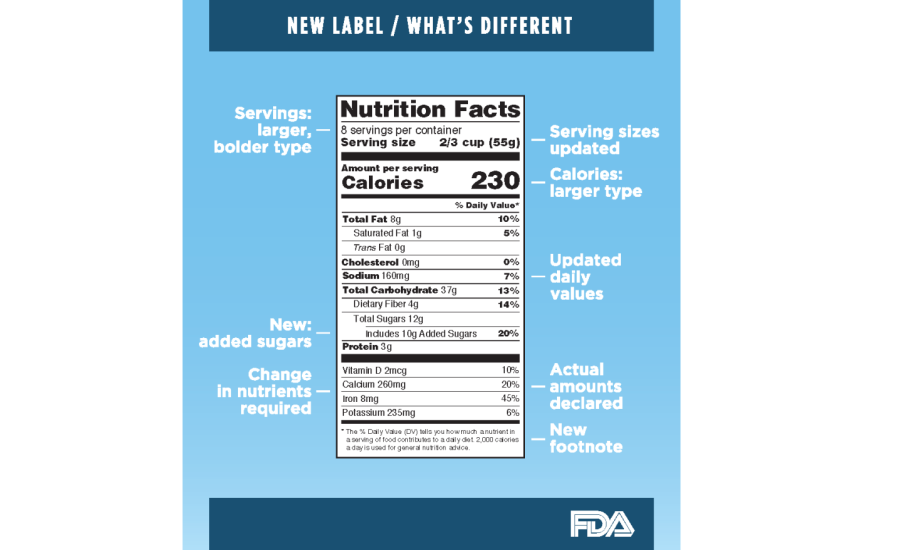
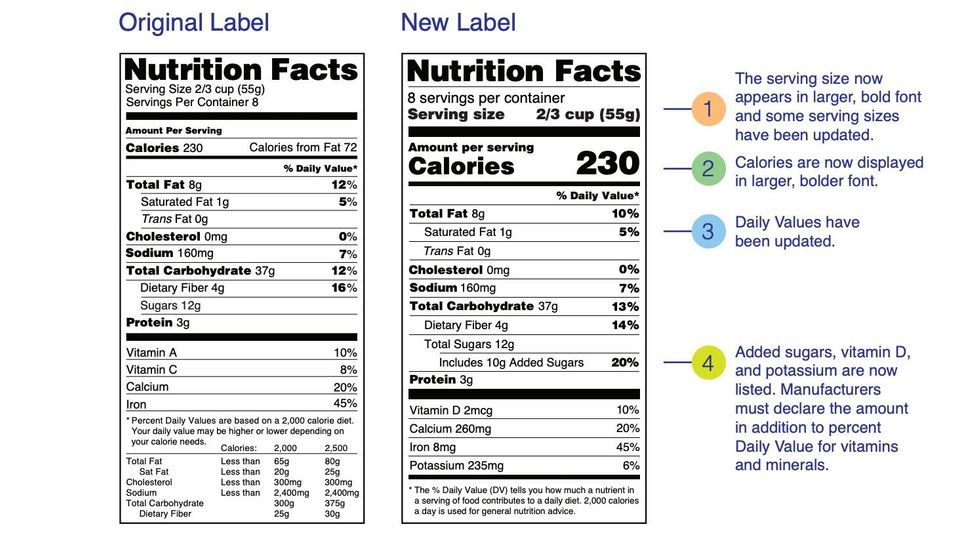
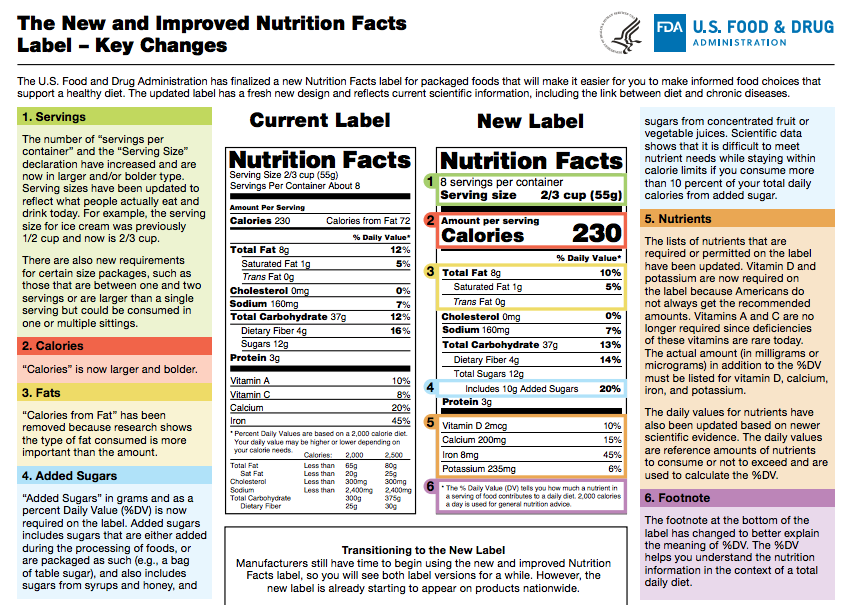
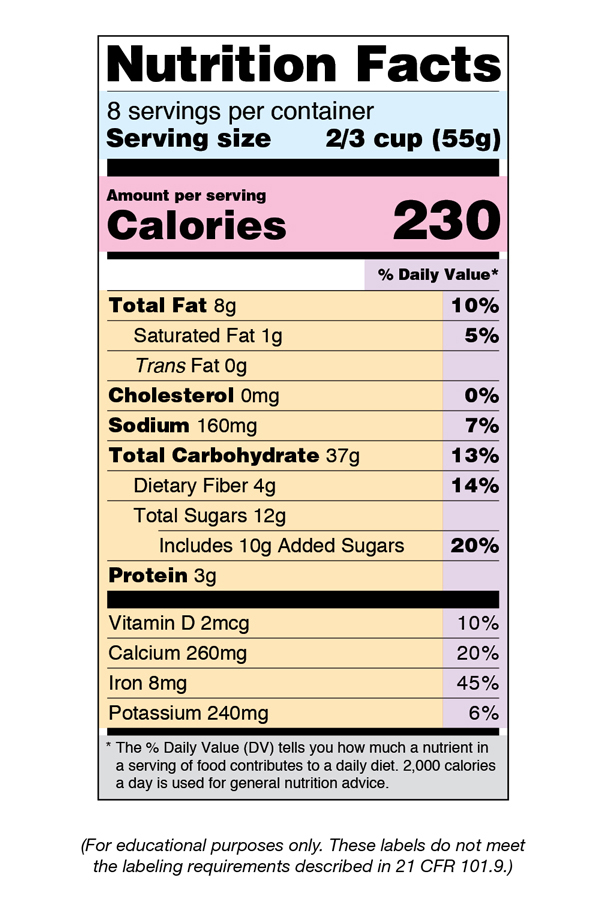
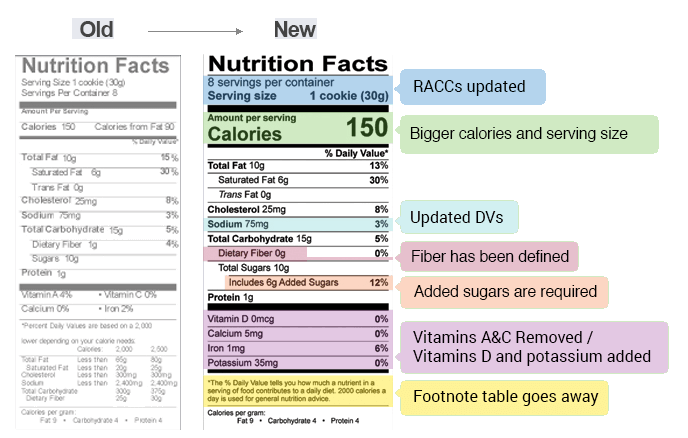
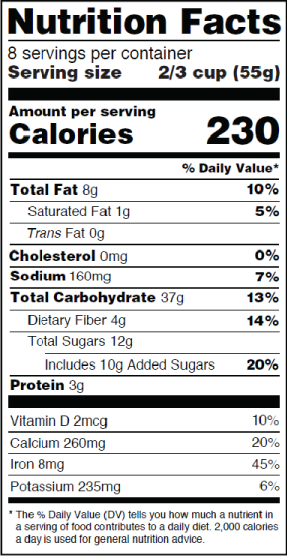
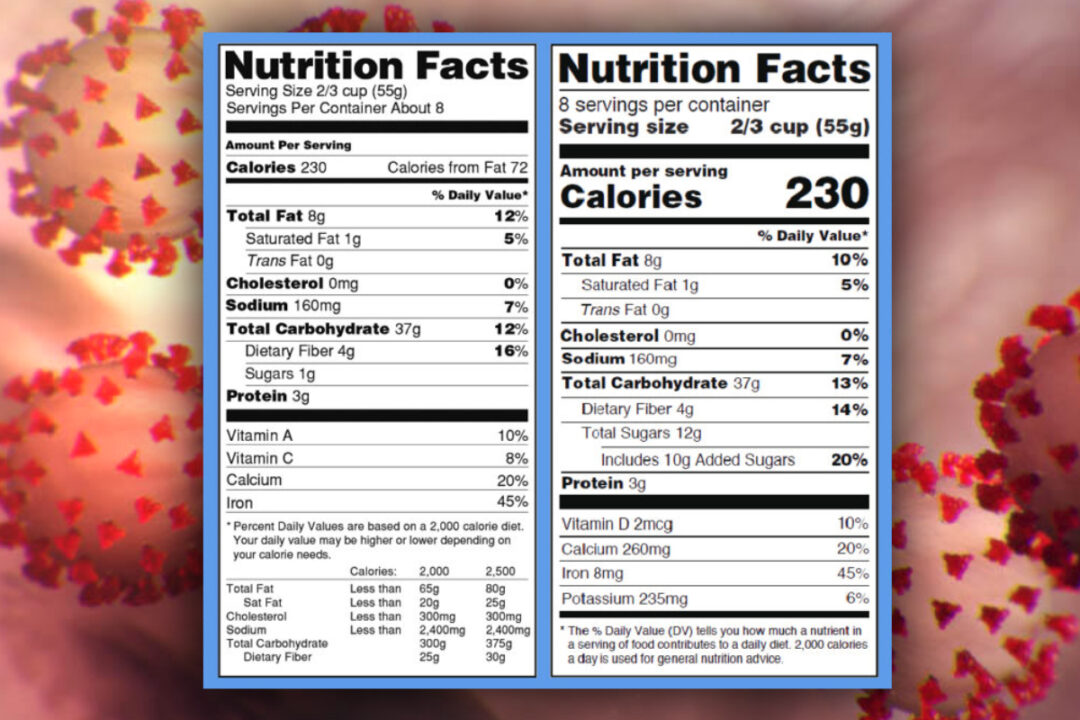
Fda requirements food labels. › consumers › consumer-updatesHave Food Allergies? Read the Label | FDA Jan 29, 2021 · Food labels can help consumers with food allergies avoid foods or ingredients that they or their families are allergic to. This is because a federal law, the Food Allergen Labeling and Consumer ... The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated... FDA Nutrition Label: What Needs to Be on Them? | Blog What Exactly is an FDA Nutrition Label? An FDA nutrition label is a transparent description and list of the ingredients in a food product. This includes everything from serving size, allergens, ingredients, and calorie count. The FDA has a strict set of guidelines and regulations that give a product FDA approval. Without an FDA nutrition label ... 2020 FDA Regulations for Food Labeling - LabelCalc 2020 FDA Regulations for Food Labeling: Are You Compliant? According to new FDA regulations regarding food labeling for food manufacturers: companies exceeding $10 million in revenue must comply with new changes by Jan 1, 2020. Companies below that revenue mark or single supply manufacturers of items such as sugar and honey have until Jan 2021.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the common or usual name of each ingredient ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a) You must take necessary actions to determine whether packaging for dietary supplements meets specifications so that the condition of the packaging will ensure the quality of your dietary... › food › food-labeling-nutritionUse of the Term Natural on Food Labeling | FDA The comment period closed May 10, 2016. View submitted comments in docket folder FDA-2014-N-1207 on Regulations.gov.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content... Food Ingredients & Packaging | FDA FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in meat, poultry, vegetables... › food › new-nutrition-facts-labelDaily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ... Guidance for Industry: Food Labeling Guide | FDA It is the responsibility for the food industry to remain current with the legal requirements for food labeling. All new regulations are published in the Federal Register (FR) prior to...
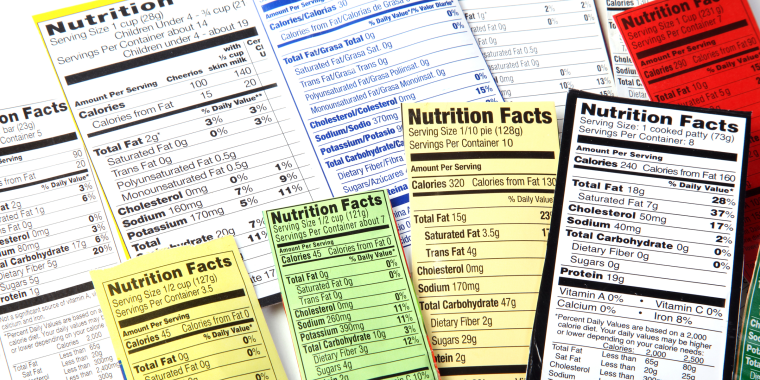
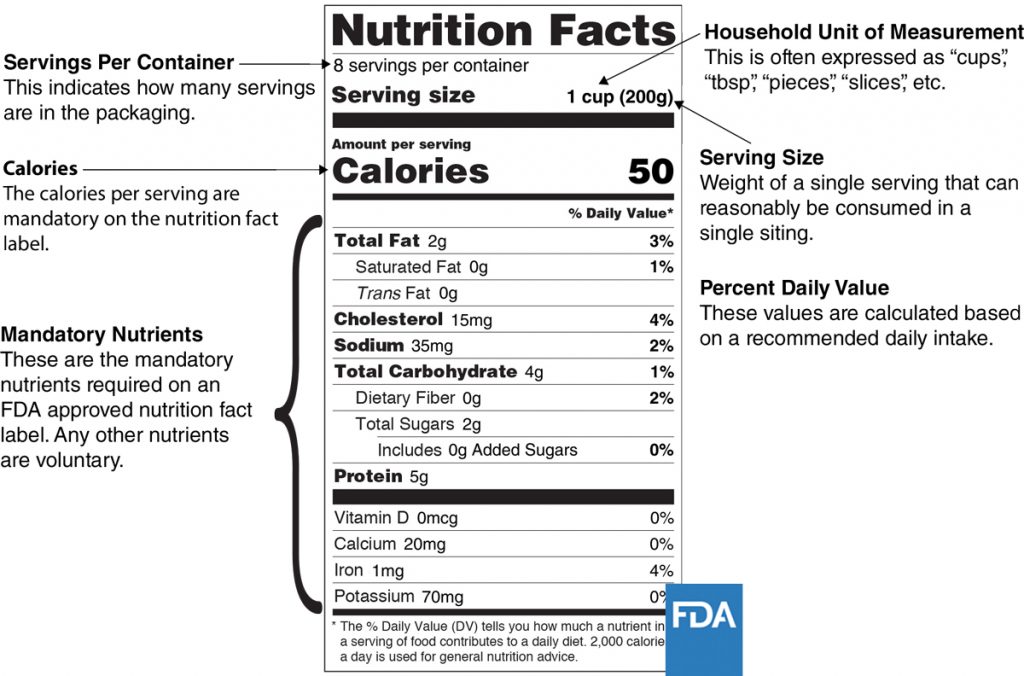
What are the Requirements for a Food Label? - Short Food Labeling Guide Required Food Label Information The FDA requires seven areas of information on food labels for legal sale of these goods. These items include the following information about the food product. All labeling must be in English, though some foreign language is appropriate so long as the English translation is also present
FDA Announces Temporary Food Labeling During COVID-19 Pandemic entitled " temporary policy regarding certain food labeling requirements during the covid-19 public health emergency: minor formulation changes and vending machines ," this guidance is one of...
What are the FDA requirements for food- USA Food regulations Labeling is one of the important FDA requirements for food products. We can offer labeling review services and our fee for per product labeling review is $ 299. In order to review the label, you should provide us label design in PDF or image format. We offer discounts on multiple labeling reviews.
Food Labeling & Nutrition | FDA 16.05.2022 · What's new in food labeling and nutrition, including label claims, nutrition labeling for restaurants, and links to industry guidance.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Requirements of conspicuousness and legibility shall include the specifications that: (1) The ratio of height to width (of the letter) shall not exceed a differential of 3 units to 1 unit (no...
› food › food-industryHow to Start a Food Business | FDA - U.S. Food and Drug ... Food manufacturers are responsible for developing labels (including nutrition information) that meet legal food labeling requirements. All labeling of FDA-regulated food products must be truthful ...
Guidance for Industry: Food Labeling Guide | FDA Under FDA's laws and regulations, FDA does not pre-approve labels for food products. Questions concerning the labeling of food products may be directed to the Food Labeling and Standards Staff ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables)...
US FDA labeling requirements for food - FDABasics Overview of US FDA labeling requirements for food P The product should bear a statement of identity (common name of the product) more prominently on the principal display panel. P Net weight should appear both in US customary and metric systems ( example in Ounce and Gram) P List of ingredients in the order of predominance by weight
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
PDF Food Labeling Guide - Food and Drug Administration Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740 (Tel)...
› food › food-labeling-nutritionFood Allergies | FDA - U.S. Food and Drug Administration Jun 23, 2022 · The FDA’s “Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food” rule (CGMP & PC rule, 21 CFR part 117) establishes requirements applicable ...
FDA Food Labeling Exemptions - LabelCalc A lot of people get tripped up on the rounding rules for the nutrition facts panel, others get confused about nutrient content claims, and some have trouble understanding label size requirements. The one thing, however, that almost all food manufacturers struggle with is understanding the FDA food labeling exemptions.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.9 Nutrition labeling of food. (a) Nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is...









![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)




![The FDA's Updated Nutrition Facts Label [Infographic]](https://www.repsly.com/hubfs/Infographics/How%20to%20design%20the%20perfect%20packaging.png)







![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f81b6e365340866e0eeb_Inner-Images-4.jpg)
Post a Comment for "40 fda requirements food labels"